The Mineral labradorite
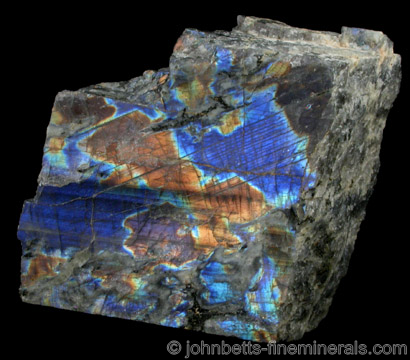
Labradorite is an unusual mineral. It can display a beautiful iridescent play of colors, caused by internal fractures in the mineral that reflect light back and forth, dispersing it into different colors. This effect, known as labradorescence, gives Labradorite its appeal and fame. Specimens sold to collectors are usually polished or sliced by dealers to fully bring out this effect. Sliced slabs are sometimes sold by dealers in water, which enhances the effect.
Labradorite belongs to the Plagioclase Feldspar group, an
isomorphous solid solution series. Albite is one member,
containing sodium and no calcium. The other end member, Anorthite,
contains calcium and no sodium. Labradorite is an intermediary
member of this series. Labradorite is considered by some authorities as a
variety of Anorthite rather then a separate mineral. The acclaimed
Dana's System of
Mineralogy lists Labradorite as an individual mineral,
whereas the IMA does not recognize it as individual mineral
species, but rather a sodium-rich variety of Anorthite.
For additional information, see the gemstone section on Labradorite.
Chemical Formula
(Ca,Na)Al1-2Si3-2O8
Color
White, gray, light blue, light green, pale orange-red, black, usually with a strong multicolored display of purple, blue, and green schillers. A variety from Finland known as Spectrolite, shows the Schiller effect with dark reds, orange, yellow, blues and green color flashes.
Properties
Streak
White |
Hardness
6 - 6.5 |
Transparency
Transparent to translucent |
Specific Gravity
2.69 - 2.72 |
Luster
Vitreous to pearly |
Cleavage
2,1 - basal ; 2,1 - prismatic ; 3,1 - pinacoidal. The cleavage angle is about 90º. |
Fracture
Conchoidal to uneven |
Tenacity
Brittle |
Crystal Habits
Labradorite rarely forms in crystals. When it does, they are generally tabular and often twinned. Most commonly occurs massive , grainy , as elongated fragments, as chunky masses, and rounded.
Varieties
-
Variety of Labradorite from Finland that displays a schiller color effect with intense colors such as dark red, orange, yellow, blue, and green.
Uses
Labradorite is a popular mineral, and it makes a unique gemstone. It is cut and polished into cabochons and beads, and occasionally as other facets. Some exquisite ornaments are carved out of large Labradorite chunks.
Noteworthy Localities
Perhaps the most colorful Labradorite comes from Finland at the Ylamaa Quarries, Lappeenranta. Two other outstanding localities are Golovinskoye, Zhytomyr, Ukraine; and the Antsohamamy Quarry, Tulear Province, Madagascar. Good Labradorite also comes from Black Hill, New South Wales, Australia.
Some of the most important Labradorite deposits are in Canada in Labrador, at Nain and Tabor Island. In the U.S., the Adirondack Mountains of upstate New York have produced colorful Labradorite, especially at Saranac Lake. Franklin Co.; Blue Ridge Road, North Hudson, Essex Co.; and Roaring Brook Falls, Keene Valley, Essex Co. A transparent form of Labradorite comes from Millard Co., Utah; and from the Woodward Ranch, near Alpine, Brewster Co., Texas.
Distingushing Similar Minerals
Due to its unique color effect, Labradorite is easily distinguished from all minerals. However, specimens that don't exhibit labradorescence may be confused with many minerals, especially other feldspars.